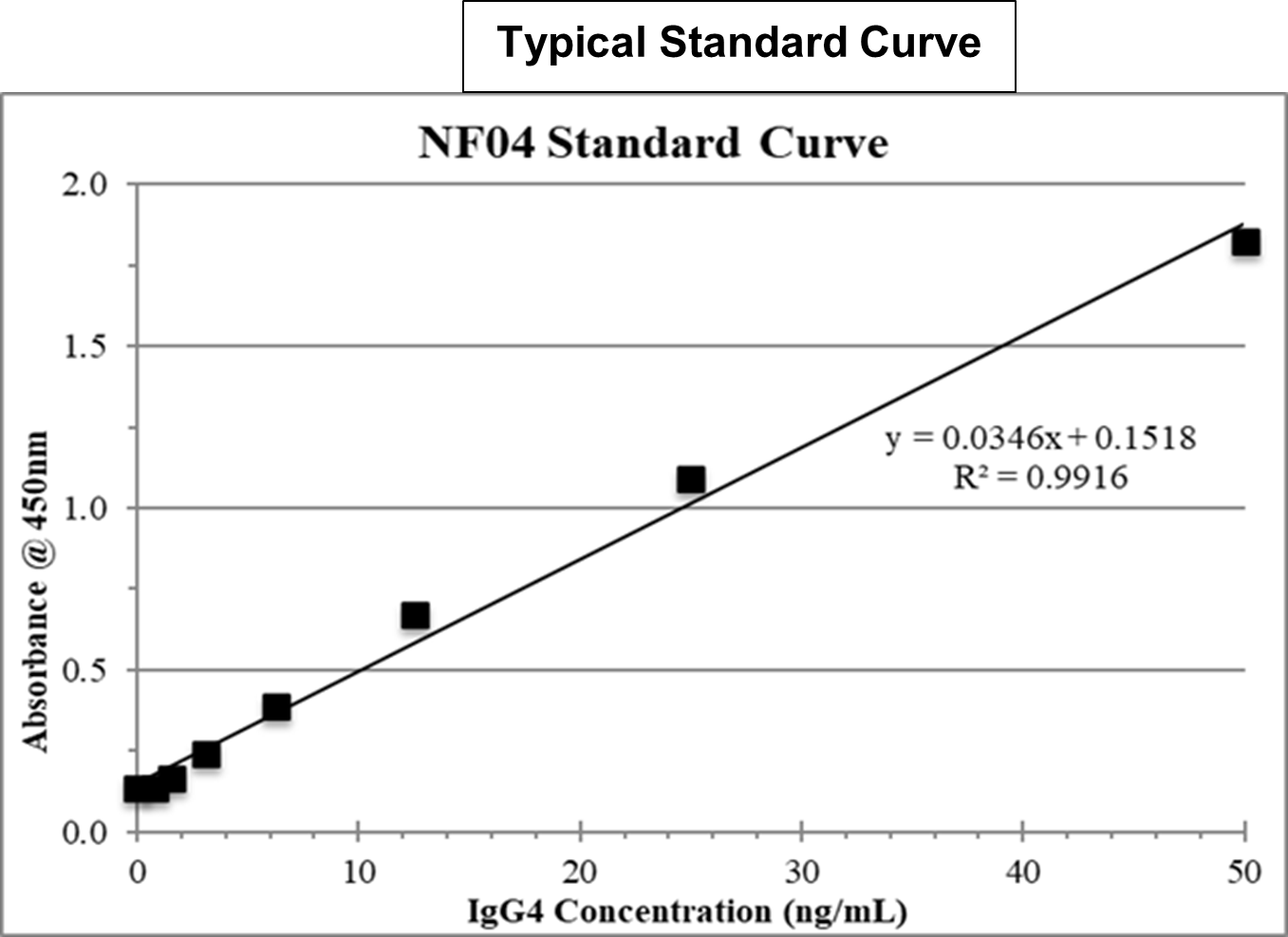
Product Overview
Recombinant human monoclonal antibodies have become a major focus of new drug discovery in the Pharmaceutical and Biotech industries, with hundreds of new human monoclonal antibodies currently in preclinical development. Fully human recombinant antibodies have gradually replaced humanized mouse or rat antibodies, with IgG1 and IgG4 as the preferred engineered isotypes2. As part of the developmental process, human therapeutic antibody drug candidate molecules must be tested in non-human primate species to assess their pharmacokinetic profile and potential toxicity3. Primate and human antibodies are highly homologous, making it difficult to distinguish the human antibody drug candidate from the endogenous primate antibodies in a serum or plasma sample by traditional human IgG-specific immunoassays. Therefore, we developed two highly sensitive ELISAs that distinguish human IgG1 or human IgG4 from rhesus or cynomolgus monkey immunoglobulins. This Recombinant (Therapeutic) Human IgG4 ELISA can be used to accurately quantify human IgG4 in the serum or plasma of rhesus or cynomolgus monkeys without cross reacting with antibodies of those species. This same ELISA can also be used to quantify human IgG4 in the plasma of other preclinical test species (mouse, rat, rabbit, guinea pig, etc.) if desired. This assay is supplied with a recombinant human IgG4 kappa standard. The customer may wish to substitute their own specific drug candidate IgG4 for this standard in the assay.
References
1. Buntz, B. (2021, May 14). 50 of 2020's best-selling pharmaceuticals. Drug Discovery and Development. Retrieved January 20, 2022, from https://www.drugdiscoverytrends.com/50-of-2020s-best-selling-pharmaceuticals/
2. Salfeld, J. (2007) Isotype selection in antibody engineering. Nat Biotechnol 25, 1369–1372.
3. Stubenrauch K, Wessels U, Lenz H.; (2009) Evaluation of an immunoassay for human-specific quantitation of therapeutic antibodies in serum samples from non-human primates. J Pharm Biomed Anal.; 49(4):1003-8
